2 experimental Ebola vaccines show promise
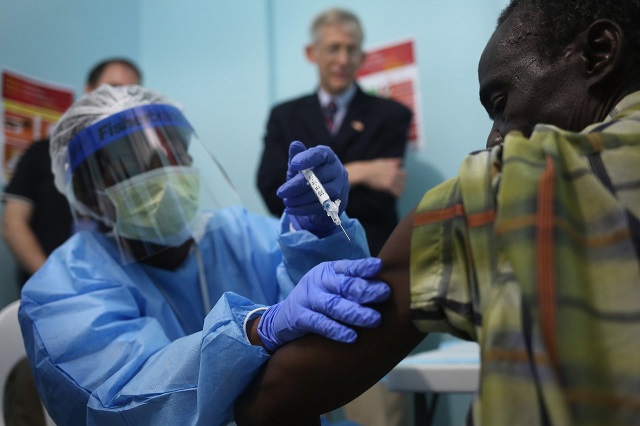
Miami — Two experimental vaccines against the Ebola virus have shown promise in protecting against the haemorrhagic fever for at least a year, according to the results of a major clinical trial published on Wednesday.
The report in the New England Journal of Medicine is the first full account of a large-scale effort to test what could be the first vaccine against Ebola.
The outbreak of the highly contagious and often deadly virus killed more than 11 000 people, mainly in Liberia, Guinea and Sierra Leone, as it swept West Africa from late 2013 to 2016. The phase II study involved 1 500 people in Monrovia, Liberia. Participants were randomly assigned to receive one of two vaccines being tested, or a placebo.
The vaccine candidates included the frontrunner, rVSV-ZEBOV, initially engineered by Canadian government scientists and now licensed to Merck, Sharp and Dohme Corporation.
The second vaccine candidate was cAd3-EBOZ, co-developed by the US National Institute for Allergy and Infectious Diseases’ (NIAID) Vaccine Research Centre and GlaxoSmithKline.
After one month, 84 percent of rVSV-ZEBOV recipients developed an antibody response. At one year, 80 percent still had this protection.
For the other candidate, cAd3-EBOZ, 71percent developed an antibody response, and 64 percent still had such a response at one year, when the trial ended.
“This clinical trial has yielded valuable information that is essential for the continued development of these two Ebola vaccine candidates and also demonstrates that well-designed, ethically sound clinical research can be conducted during an epidemic,” said NIAID director Anthony Fauci. — Al Jazeera










Comments